The basic principle of Bacterial Endotoxin Test can make it the most sensitive test that one can use to detect and quantify endotoxins, toxins which are famously known for causing fever in humans.
For Injections in cartridges or prefilled syringes, assemble the container with any necessary components like a needle or plunger. Following exactly the same process as previously mentioned, and without the need of emptying the needle, transfer your complete contents of each container to some dry, tared beaker by slowly and gradually and regularly depressing the plunger.
4. Can finished product samples for Examination of bacterial endotoxins be pooled into a composite sample ahead of analysis?
Packaging and Storage The amount of injection in single-dose containers offers the quantity specified for parenteral administration at just one time and in no scenario is greater than ample to permit the withdrawal and administration of one L.
While this guidance just isn't intended to deal with biological assays, most of the principles in the steerage are applicable to bacterial endotoxins testing. We update assistance files periodically. To be sure to have the most recent Model of a direction, Look at the FDA Drugs assistance Web content at
are Section of the outer membrane in the mobile wall of Gram-negative microbes; and they're invariably connected with Gram-destructive microbes whether the organisms are pathogenic or not.
These a few paperwork describe the fundamental rules on the gel clot, photometric, and kinetic test strategies, and advocate that appropriate parts and finished goods be tested for your existence of pyrogens and endotoxins.
For giant-quantity intravenous answers, choose 1 container, and transfer the contents right click here into a dry measuring cylinder of these types of sizing that the amount to become calculated occupies no less than forty% of its rated volume. The volume isn't under the labeled quantity.
Furthermore, raw materials and final item must even be tested with the presence of bacterial endotoxins. Lonza's wide range of pyrogen and endotoxin testing alternatives supports your endeavours in testing, such as for vaccines, cell and gene therapies and biologics.
Nonetheless, from the curiosity of assuring the quality of injection preparations as they are literally administered, the subsequent nondestructive tests are supplied for demonstrating the suitability of constituted alternatives when they're ready just just before use.
In the course of the same surgical method or placement in the exact same surgical click here web-site, many units of the exact same unit from one particular producer should really commonly meet up with the exact same endotoxins limit as just one device administered through the method.
Sample template on how to produce your research achievements and final results when implementing for just a fellowship or grant
Nevertheless, as the compendial chapters and benchmarks usually do not address sure regulatory perspectives, FDA is giving supplemental info in this steering to elucidate our present-day thinking concerning the submission and upkeep of pyrogen and endotoxins testing for FDA-controlled merchandise.
Beneficial Manage has to be incorporated to verify that it is acceptable to utilize the parameters of a previous (archived) standard curve to compute endotoxin concentrations.
 Spencer Elden Then & Now!
Spencer Elden Then & Now! Barret Oliver Then & Now!
Barret Oliver Then & Now! Andrew Keegan Then & Now!
Andrew Keegan Then & Now!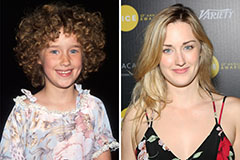 Ashley Johnson Then & Now!
Ashley Johnson Then & Now! Dawn Wells Then & Now!
Dawn Wells Then & Now!